 Multiple Choice Questions
Multiple Choice QuestionsActivation energy (Ea) and rate constants (k1 and k2) of chemical reaction at two different temperatures (T1 and T2) are related by




D.

According to Arrhenius equation, activation energy (Ea) and rate constants (k1 and k2) of chemical reaction at two different temperatures (T1 and T2) are related as,
Standard reduction potentials of the half-reactions are given below.
F2 (g) +2e- → 2F- (aq) ; Eo = +2.85 V
Cl2 (g) +2e- →2Cl- (aq) ; Eo = +1.36V
Br2 (l) +2e- → 2Br- (aq) ; Eo = +1.06 V
I2 (s) +2e- →2I- (aq); Eo = +0.53 V
The strongest oxidising and reducing agents respectively are
F2 and I-
Br2 and Cl-
Cl2 and Br-
Cl2 and Br-
Vapour pressure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 25oC are 200 mmHg and 41.5 mmHg respectively, Vapour pressure of the solution obtained by mixing 25.5 g of CHCl3 and 40 g of CH2Cl2 at the same temperature will be
(molecular mass of CHCl3= 119.5 u and molecular mass of CH2Cl2 = 85 u)
173.9 mmHg
615.0 mmHg
347.9 mmHg
347.9 mmHg
Molar conductivities (Λom) at infinite dilution of NaCl, HCl and CH3COONa are 126.4, 425.9 and 91.0 S Cm2 mol-1 respectively. Λom for CH3COOH will be
425.5 S cm2 mol-1
180.5 S cm2 mol-1
290.8 S cm2 mol-1
290.8 S cm2 mol-1
Which one of the following does not correctly represent the correct order of the property indicated aginst it?
Ti < V<Cr< Mn: increasing number of oxidation states
Ti< V<Cr3+<Mn3+ : increasing magnetic moment
Ti < V < Cr < Mn : Increasing melting points
Ti < V < Cr < Mn : Increasing melting points
Four successive members of the first series of the transition metals are listed below. For which one of them, the standard potential value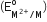 has a positive sign?
has a positive sign?
Co (Z=27)
Ni (Z=28)
Cu (Z=29)
Cu (Z=29)
In Which of the following arrangement, the given sequence is not strictly according to the property indicated against it ?
HF < HCl< HBr < HI : increasing acidic strength
H2O < H2S < H2Se < H2Te : increasing pKa values.
NH3 < PH3 < AsH3 < SbH3 : increasing acidic character
NH3 < PH3 < AsH3 < SbH3 : increasing acidic character
The catalytic activity of transition metals and their compounds is ascribed mainly to
their magnetic behaviour
their unfilled d- orbitals
their ability to adopt variable oxidation states
their ability to adopt variable oxidation states
The Gibb's energy for the decomposition of Al2O3 at 500o C is as follow
2/3 Al2O3 → 4/3 Al + O2;
ΔrG = +960 kJ mol-1
The potential difference needed for the electrolytic reduction aluminium oxide (Al2O3) at 5000 C is at least
4.5 V
3.0 V
2.5 V
2.5 V
