 Multiple Choice Questions
Multiple Choice QuestionsAddition of sodium thiosulphate solution to a solution of silver nitrate gives X as white precipitate, insoluble in water but soluble in excess thiosulphate solution to give Y. On boiling in water, Y gives Z. X, Y and Z respectively are
Ag2S2O3, Na3[Ag(S2O3)2], Ag2S
Ag2SO4, Na[Ag(S2O3)2], Ag2S2
Ag2S2O3, Na5[Ag(S2O3)3], AgS
Ag2SO3, Na3[Ag(S2O3)2], Ag2O
A.
Ag2S2O3, Na3[Ag(S2O3)2], Ag2S
X, Y and Z are Ag2S2O3, Na3[Ag(S2O3)2], Ag2S.
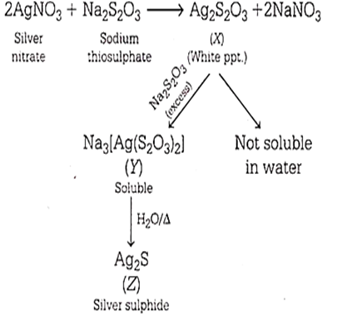
Hence, X = Ag2S2O3
Y = Na3[Ag(S2O3)2]
Z = Ag2S
Optical isomerism is exhibited by (ox= oxalate anion; en= ethylenediamine).
cis-[CrCl2(ox)2]3-
[Co(en)3]3+
trans-[CrCl2(ox)2]3-
[Co(ox) (en)2]+
Extraction of gold (Au) involves the formation of complex ions X and Y.
Gold ore HO- + X Y + Au
X and Y respectively are
AuCN and Zn(CN)
Au(CN) and Zn(CN)
Au(CN)
Au(CN)
Sulphuryl chloride (SO2Cl2) reacts with white phosphorus (P4) to give
PCl5, SO2
OPCl3, SOCl2
PCl5, SO2, S2Cl2
OPCl3, SO2, S2Cl2
In the following compound, the number of sp-hybridised carbons are
CH2 = C = CH - CN(CH) - C ≡ CH
2
3
4
5
For the reaction A + 2B → C, the reaction rate is doubled, if the concentration of A is doubled. The rate is increased by four times when concentrations of both A and B are increased by four times. The order of the reaction is
3
0
1
2
Suppose the mass of a single Ag-atom is m. Ag metal crystallises in fcc lattice with unit cell of length a. The density of Ag metal in terms of a and 'm' is
At a particular temperature, the ratio of equivalent conductance to specific conductance of a 0.01 N NaCl solution is
105 cm3
103 cm3
10 cm3
105 cm2
