 Multiple Choice Questions
Multiple Choice QuestionsAt temperature of 298K, the emf of the following electrochemical cell,
Ag (s) | Ag+ (0.1 M)|| Zn2+ (0.1 M) | Zn (s) will be (Given, E°cell = -1.562 V)
-1.532 V
-1.503 V
1.532 V
-3.06 V
A.
-1.532 V
From the given cell, the cell reaction is
2Ag (s) + Zn2+ (0.1M) → 2Ag+ (0.1 M) + Zn (s)
The Nernst equation is
Ecell = E°cell -
Roasted copper pyrite on smelting with sand produces
FeSiO3 as fusible slag and Cu2S as matte
CaSiO3 as infusible slag and Cu2O as matte
Ca3(PO4)2 as fusible slag and Cu2S as matte
Ca3(PO4)2 as infusible slag and Cu2S as matte
Ionisation potential values of noble gases decrease down the group with increase in atomic size. Xenon forms binary fluorides by the direct reaction of elements. Identify the correct statement(s) from below.
Only the heavier noble gases form such compounds
It happens because the noble gases have higher ionisation energies
It happens because the compounds are formed with electronegative ligands
Octet of electrons provide the stable arrangements
The increase in rate constant of a chemical reaction with increasing temperature is (are) due to the fact(s) that
the number of collisions among the reactant molecules increases with increasing temperature
the activation energy of the reaction decreases with increasing temperature
the concentration of the reactant molecules increases with increasing temperature
the number of reactant molecules acquiring the activation energy increases with increasing temperature
In a mixture, two enantiomers are found to be present in 85% and 15% respectively. The enantiomeric excess (ee) is
85%
15%
70%
60%
Best reagent for nuclear iodination of aromatic compounds is
KI/ CH3COCH3
I2/CH3CN
KI/ CH3COOH
I2 / HNO3
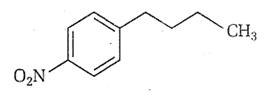
Identify the correct method for the synthesis of the compound shown below from the following alternatives.
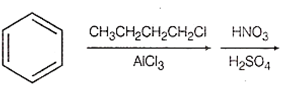
![]()
![]()
![]()
