 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following compounds is aromatic in nature?
![]()
![]()
![]()
![]()
A.
![]()
Among all the given options, option a is correct.
It is due to the presence of (4n + 2) e- it follows Huckel's rule and therefore, it is aromatic.
In option b, due to te presence of extra lone pair of electrons, total e- comes out to be 4e-. Thus, it is anti-aromatic.
In option c, although it is cyclic and has conjugated 8e- but Huckel's (4n + 2) rule is not followed and also ring is not planar. Hence, it is non- aromatic.
In option d, it has 6e- in conjugation but not in the ring, hence it is non - aromatic.
What is the correct order of basicity among the following compounds ?
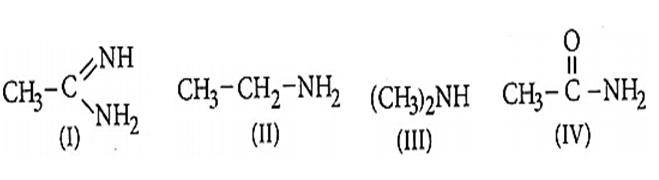
II > I > III > IV
I > II > III > IV
III > I > II > IV
I > III > II > IV
Select the correct options from the following :
Graphene is an atomic layer of graphite
Graphene is an atomic layer composed of sp2-hybridised carbon.
Chemical bonds in graphite are similar in strength to that of diamond.
All of these.
Arrange the following nucleophiles in the decreasing order of nucleophilicity :
(A) CH3COO-
(B) CH3O-
(C) CN-
(D) 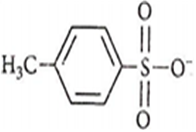
C, B, A, D
A, B, C, D
C, C, B, A
B, C, A, D
Assertion : The carbonate of lithium decomposes easily on heating to form lithium oxide and CO2.
Reason : Lithium being very small in size polarises large carbonate ion leading to the formation of more stable Li2O and CO2.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false
If both assertion and reason are false
Assertion : Thiophene present in commercial benzene as an impurity can be removed by shaking the mixture with cold concentrated H2SO4.
Reason : Thiophene is a heterocyclic aromatic compound.
If both assertion and reason are true and reason is the correct explanation of assertion
If both assertion and reason are true but reason is not the correct explanation of assertion
If assertion is true but reason is false
If both assertion and reason are false.
Calculate (in S cm2 mol-1) using appropriate molar conductances of the electrolytes listed in the given table
| Electrolyte | KCl | KNO3 | HCl | NaOAc | NaCl |
|
(S cm2 mol-1) |
149.9 | 145.0 | 426.2 | 91.0 | 126.5 |
at infinite dilution in H2O at 25C
517.2
305.0
390.7
217.5
