 Short Answer Type
Short Answer TypeArrange the following in order of decreasing basic strength:
(CH3)3(CO–, CH3O–, (CH3)2CHO–
Compare and explain the relative Bronsted basicities of 1°, 2° and 3° alcohols in the liquid state.
Give one example of a reaction to show that alcohol acts as a Bronsted base.
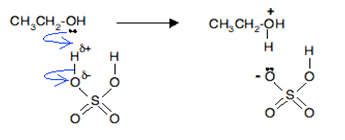
Explain why sodium (Na) may be used to remove the last trates of H2O from benzene but not from ethanol?
Give the major product of the reaction of ethanol with:
(i) Ethanoic anhydride (CH3CO)2O.
(ii) Ethanol chloride in the presence of pyridine.
