 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWrite the mechanism of hydration of ethene to yield ethanol.
The mechanism of the reaction involves the following three step:
Step 1: Proptonation of ethene to form carbocation by electrophilic attack of H3O+.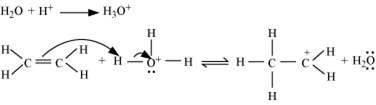
Step 2: Nucleophilic attack of water on carbocation.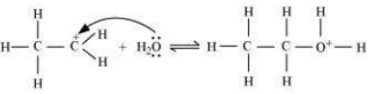
Step 3: Deprotonation to form an ethanol.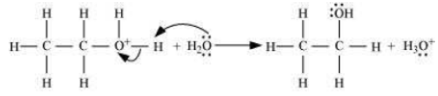
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeGive two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of control?
 Short Answer Type
Short Answer Type