 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeGive two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of control?
 Short Answer Type
Short Answer TypeThe -OH group attached to the benzene ring activates it towards electrophillic substitution reaction. It direct the incoming group to ortho and para position in the ring as these positions become electron rich due to the resonance effect the caused by -OH group.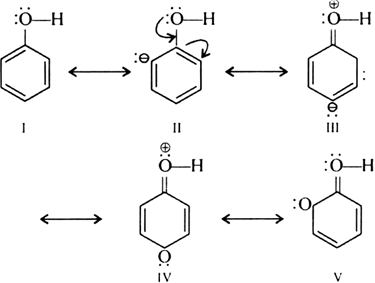
Since there is –ve charge at o and p position. Therefore, —OH group activates benzene ring towards electrophilic substitution reaction.
