Multiple Choice Questions
Multiple Choice QuestionsPhenol on treatment with CO2 in the presence of NaOH followed by acidification produces compound X as the major product. X on treatment with (CH3CO)2O in the presence of catalytic amount of H2SO4 produces:
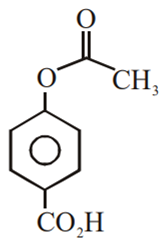
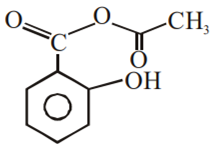
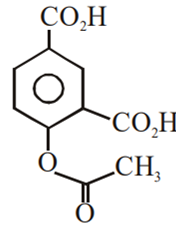
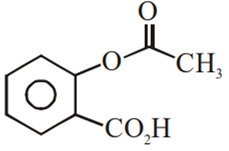
Which of the following compounds would not react with Lucas reagent at room temperature?
C6H5CH2OH
CH3CH2CH2OH
(CH3)3COH
Which of the following will be dehydrated most readily in alkaline medium?
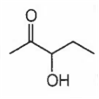
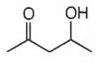
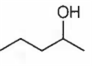
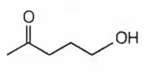
B.

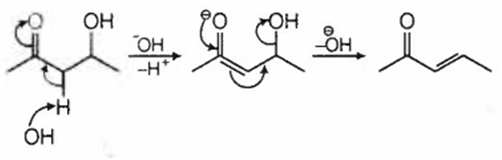
In alkaline medium the acidic proton present in the molecule is abstracted to give enolate ion. In the next step hydroxide ion if present at β- position leaves giving α- β- unsaturated carbonyl compound. In (a), (c) and (d), dehydration cannot take place due to improper placement of groups.
The compound that would produce a nauseating smell/ odour with a hot mixture of chloroform and ethanolic potassium hydroxide is
PhCONH2
PhNHCH3
PhNH2
PhOH
The correct statement regarding the following compounds is
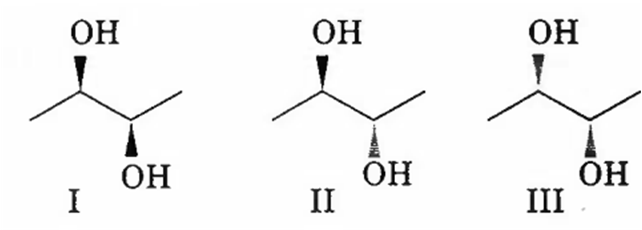
all three compounds are chiral
only I and II are chiral
I and III are diastereomers
only I and III are chiral
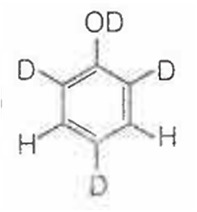
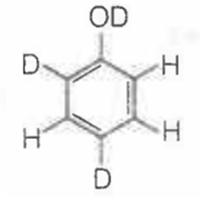
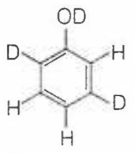
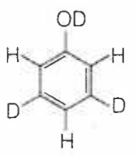
When phenol is treated with D2SO4 /D2O, some of the hydrogens get exchanged.The final product in this exchange reaction is