Multiple Choice Questions
Multiple Choice QuestionsPhenol on treatment with CO2 in the presence of NaOH followed by acidification produces compound X as the major product. X on treatment with (CH3CO)2O in the presence of catalytic amount of H2SO4 produces:
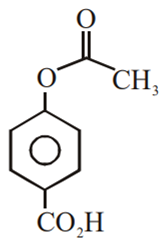
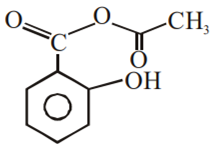
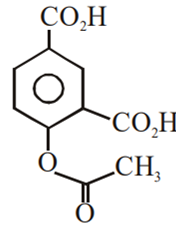
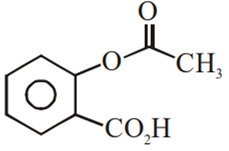
Which of the following compounds would not react with Lucas reagent at room temperature?
C6H5CH2OH
CH3CH2CH2OH
(CH3)3COH
The compound that would produce a nauseating smell/ odour with a hot mixture of chloroform and ethanolic potassium hydroxide is
PhCONH2
PhNHCH3
PhNH2
PhOH
The correct statement regarding the following compounds is
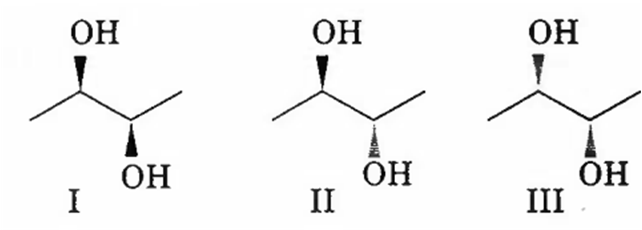
all three compounds are chiral
only I and II are chiral
I and III are diastereomers
only I and III are chiral
D.
only I and III are chiral
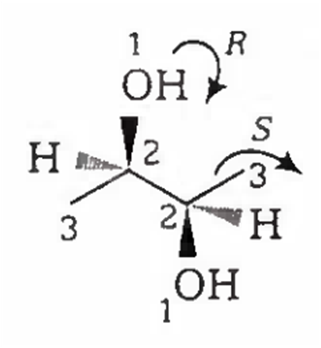 2-R, 3-R configuration, so it is a chiral compound.
2-R, 3-R configuration, so it is a chiral compound.
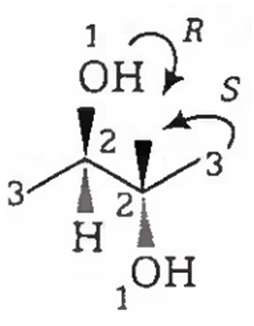 2-R, 3-S configuration, i.e., it has plane of symmetry or in other words, rotation of half part is cancelled by the other half, so it is not a chiral compound.
2-R, 3-S configuration, i.e., it has plane of symmetry or in other words, rotation of half part is cancelled by the other half, so it is not a chiral compound.
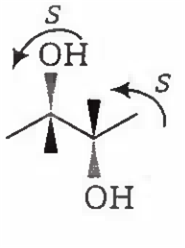 2-S, 3-S configuration, so it is also a chiral compound.
2-S, 3-S configuration, so it is also a chiral compound.
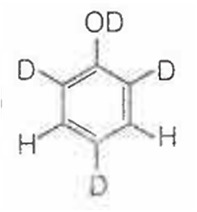
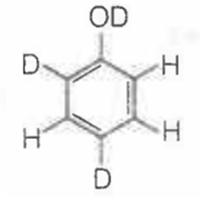
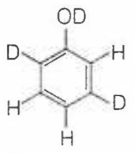
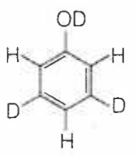
When phenol is treated with D2SO4 /D2O, some of the hydrogens get exchanged.The final product in this exchange reaction is