Multiple Choice Questions
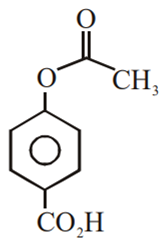
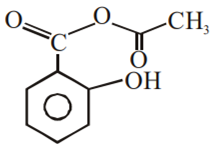
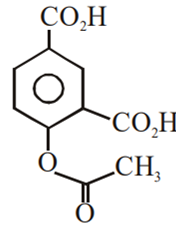
Multiple Choice QuestionsPhenol on treatment with CO2 in the presence of NaOH followed by acidification produces compound X as the major product. X on treatment with (CH3CO)2O in the presence of catalytic amount of H2SO4 produces:
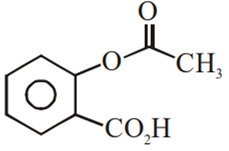
Which of the following compounds would not react with Lucas reagent at room temperature?
C6H5CH2OH
CH3CH2CH2OH
(CH3)3COH
The compound that would produce a nauseating smell/ odour with a hot mixture of chloroform and ethanolic potassium hydroxide is
PhCONH2
PhNHCH3
PhNH2
PhOH
The correct statement regarding the following compounds is
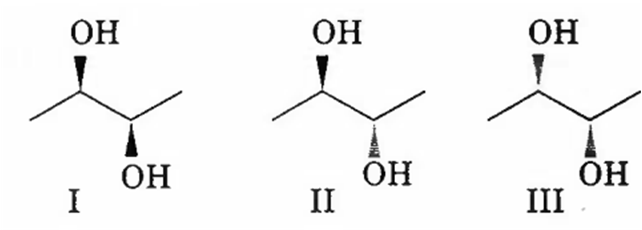
all three compounds are chiral
only I and II are chiral
I and III are diastereomers
only I and III are chiral
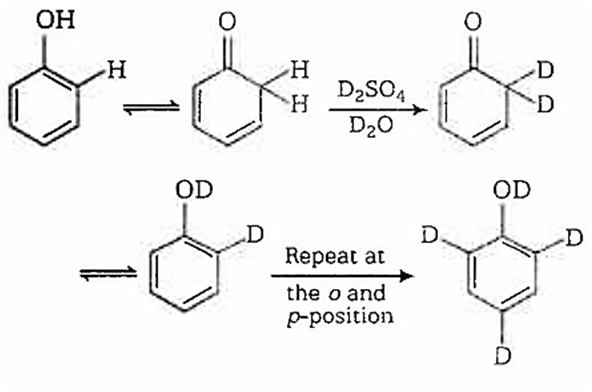
When phenol is treated with D2SO4 /D2O, some of the hydrogens get exchanged.The final product in this exchange reaction is
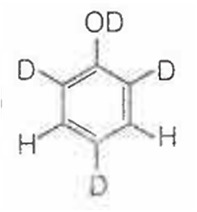
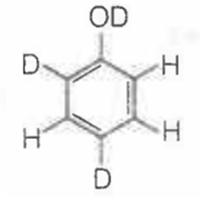
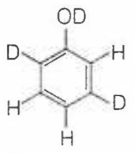
A.

In acidic medium, phenol exists in following resonating structures.
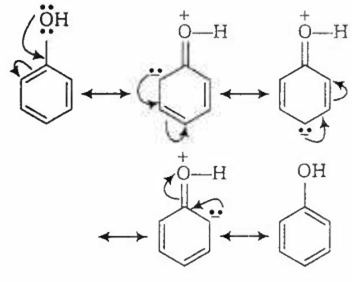
Since, o/p positions are electron rich sites, so electrophile will attack on these site, i.e., hydrogen of these sites get exchanged by D (deuterium). Hence, the final product of the reaction is 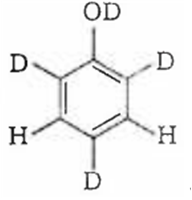 which is formed as
which is formed as