 Short Answer Type
Short Answer TypeAccount for the following:
(i) CH3CHO is more reactive than CH3 COCH3 towards reaction with HCN.
(ii) The carboxylic acid is a stronger acid than phenol.
Write the chemical equations to illustrate the following name reactions:
(i) Wolff-Kishner reduction
(ii) Aldol condensation
(iii) Cannizzaro reaction Long Answer Type
Long Answer TypeHow will you bring about the following conversions?
(i) Propanone to propane
(ii) Benzoyl chloride to benzaldehyde
(iii) Ethanal to but-2-enal
 Short Answer Type
Short Answer TypeIllustrate the following name reaction giving suitable example in each case:
(i) Clemmensen reduction
(ii) Hell-Volhard-Zelinsky reaction
(i) Clemmensen Reduction
The carbonyl group of aldehydes and ketones is reduced to the CH2 group on treatment with zinc amalgam and concentrated hydrochloric acid. This is known as Clemmensen reduction.
![]()
(ii) Hell-Volhard-Zelinsky (HVZ )reaction.
Carboxylic acids having a  hydrogen are halogenated at the
hydrogen are halogenated at the  position on treatment with chlorine or bromine in the presence of small amount of red phosphorus to give
position on treatment with chlorine or bromine in the presence of small amount of red phosphorus to give  halocarboxylic acids. The reaction is known as Hell-Volhard-Zelinsky reaction.
halocarboxylic acids. The reaction is known as Hell-Volhard-Zelinsky reaction.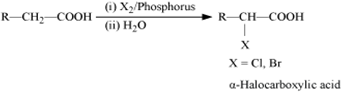
Give simple tests to distinguish between the following pairs of compounds.
(i) Pentan-2-one and Pentan-3-one
(ii) Benzaldehyde and Acetophenone
(iii) Phenol and Benzoic acid
Illustrate the following reactions giving a suitable example for each.
(i) Cross aldol condensation
(ii) Decarboxylation
Give simple tests to distinguish between the following pairs of compounds
(i) Pentan-2-one and Pentan-3-one
(ii) Benzaldehyde and Acetophenone
(iii) Phenol and Benzoic acid
Explain the following giving one example for each:
(i) Reimer-Tiemann reaction.
(ii) Friedel Craft’s acetylation of anisole.
 Long Answer Type
Long Answer TypeGive chemical tests to distinguish between
(i) Propanal and propanone,
(ii) Benzaldehyde and acetophenone.
(b) How would you obtain
(i) But-2-enal from ethanal,
(ii) Butanoic acid from butanol,
(iii) Benzoic acid from ethylbenzene?
