 Short Answer Type
Short Answer TypeAccount for the following:
(i) CH3CHO is more reactive than CH3 COCH3 towards reaction with HCN.
(ii) The carboxylic acid is a stronger acid than phenol.
Write the chemical equations to illustrate the following name reactions:
(i) Wolff-Kishner reduction
(ii) Aldol condensation
(iii) Cannizzaro reaction Long Answer Type
Long Answer TypeHow will you bring about the following conversions?
(i) Propanone to propane
(ii) Benzoyl chloride to benzaldehyde
(iii) Ethanal to but-2-enal
 Short Answer Type
Short Answer TypeIllustrate the following name reaction giving suitable example in each case:
(i) Clemmensen reduction
(ii) Hell-Volhard-Zelinsky reaction
Give simple tests to distinguish between the following pairs of compounds.
(i) Pentan-2-one and Pentan-3-one
(ii) Benzaldehyde and Acetophenone
(iii) Phenol and Benzoic acid
Illustrate the following reactions giving a suitable example for each.
(i) Cross aldol condensation
(ii) Decarboxylation
Give simple tests to distinguish between the following pairs of compounds
(i) Pentan-2-one and Pentan-3-one
(ii) Benzaldehyde and Acetophenone
(iii) Phenol and Benzoic acid
i) Pentan-2-one and pentan-3-one can be distinguished by iodoform test.
Pentan-2-one is a methyl ketone. Thus, it responds to this test. But pentan-3-one not being a methyl ketone does not respond to this test.
ii) Benzaldehyde (C6H5CHO) and acetophenone (C6H5COCH3) can be distinguished by iodoform test. Acetophenone, being a methyl ketone on treatment with I2/NaOH undergoes iodoform reaction to give a yellow ppt. of iodoform. On the other hand, Benzaldehyde does not give this test.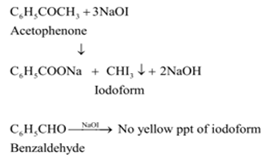
iii) Phenol and benzoic acid can be distinguished by ferric chloride test.
Ferric chloride test:
Phenol reacts with neutral FeCl3 to form ferric phenoxide complex giving violet coloration.
6C6H5OH + FeCl3 ---> [Fe (OC6H5)6]3-+3H++3Cl-
Phenol iron-phenol complex
(Violet color)
But benzoic acid reacts with neutral FeCl3 to give a buff-coloured precipitate of ferric benzoate.![]()
Explain the following giving one example for each:
(i) Reimer-Tiemann reaction.
(ii) Friedel Craft’s acetylation of anisole.
 Long Answer Type
Long Answer TypeGive chemical tests to distinguish between
(i) Propanal and propanone,
(ii) Benzaldehyde and acetophenone.
(b) How would you obtain
(i) But-2-enal from ethanal,
(ii) Butanoic acid from butanol,
(iii) Benzoic acid from ethylbenzene?
