 Long Answer Type
Long Answer Type(a) Describe the following giving linked chemical equations:
(i) Cannizzaro reaction
(ii) Decarboxylation
(b) Complete the following chemical equations:
A compound 'A' of molecular formula C2H3OCl undergoes a series of reactions as shown below. Write the structures of A, B, C and D in the following reactions:
![]()
(b) Distinguish between the following:
(i) C6H5-COCH3 and C6H5 - CHO
(ii) Benzoic acid and methyl benzoate
(c) Write the structure of 2- methylbutanal.
(a) Write the structures of the main product when acetone ( CH3-CO-CH3) reacts with the following reagent:
(i) Zn-Hg/conc. HCl
(ii) H2N-NHCONH2/H+
(iii) CH3MgBr and then H3O+
(a) Arrange the following in the increasing order of their boiling point:
C2H5OH, CH3-CHO, CH3-COOH
(b) Give a simple chemical test to distinguish between the following pair of compounds:
CH3CH2CHO and CH3CH2COCH3
 Short Answer Type
Short Answer TypeWrite the equations involved in the following reactions:
(i) Reimer - Tiemann reaction
(ii) Williamson’s ether synthesis
 Long Answer Type
Long Answer Type(a) How will you convert the following?
(i) Propanone to Propan-2-ol
(ii) Ethanal to 2-hydroxy propanoic acid
(iii) Toluene to benzoic acid
(b) Give simple chemical test to distinguish between:
(i) Pentan-2-one and Pentan-3-one
(ii) Ethanal and Propanal
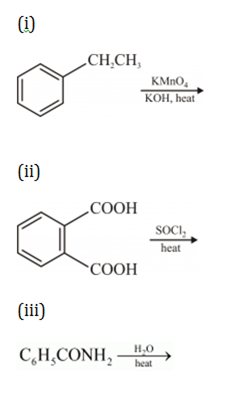
(a) Write the products of the following reactions:
(b) Which acid of each pair shown here would you expect to be stronger?
(i) F — CH2 —COOH or Cl — CH2 — COOH
(ii) 
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type(a) Write a suitable chemical equation to complete each of the following transformations:
(i) Butan-1-ol to butanoic acid
(ii) 4-methylacetophenone to benzene-1, 4-dicarboxylic acid
(b) An organic compound with molecular formula C9H10O forms 2, 4-DNP derivative, reduces Tollen’s reagent and undergoes Cannizzaro’s reaction. On vigorous oxidation, it gives 1, 2-benzenedicarboxylic acid. Identify the compound.
(a) Give chemical tests to distinguish between
(i) Propanol and propanone
(ii) Benzaldehyde and acetophenone
(b) Arrange the following compounds in an increasing order of their property as indicated:
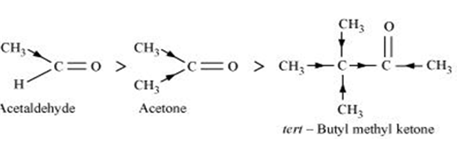
(i) Acetaldeyde, Acetone, Methyl tert-butyl ketone (reactivity towards HCN)
(ii) Benzoic acid, 3, 4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength).
(iii) CH3CH2CH (Br) COOH, CH3CH (Br) CH2COOH, (CH3)2CHCOOH (acid strength)
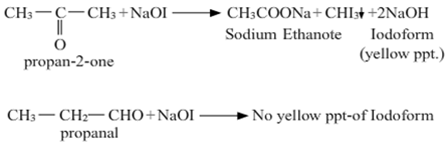
(a)
(i) Propanone gives iodoform test while propanol does not give this test.
(ii) Benzaldehyde (C6H5CHO) and acetophenone (C6H5COCH3) can be distinguished by iodoform test.
Acetophenone, being a methyl ketone on treatment with I2/NaOH undergoes iodoform reaction to give a yellow ppt. of iodoform. On the other hand, benzaldehyde does not give this test.
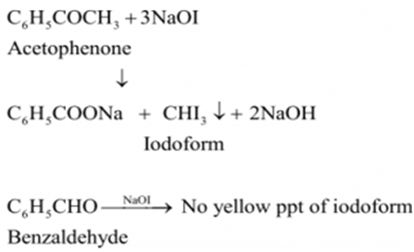
(b)
(i) Methyl tert-butyl ketone < Acetone < Acetaldehyde
When HCN reacts with a compound, the attacking species is a nucleophile, CN-.Therefore, as the negative charge on the compound increases, its reactivity with HCN decreases. In the given compounds, the +I effect increases as shown below. It can be observed that steric hindrance also increases in the same.
(ii) 4-Methoxybenzoic acid < Benzoic acid < 3, 4-Dinitrobenzoic acid
Electron-donating groups decrease the strengths of acids, while electron-withdrawing groups increase the strengths of acids. As methoxy group is an electron-donating group, 4-ethoxybenzoic acid is a weaker acid than benzoic acid. Nitro group is an electron-withdrawing group and will increase the strengths of acids.
(iii) (CH3)2 CHCOOH < CH3CH(Br)CH2COOH < CH3CH2CH(Br)COOH
After losing a proton, carboxylic acids gain a negative charge as shown:
R – COOH ---> R - COO- + H+
Now, any group that will help stabilise the negative charge will increase the stability of the carboxyl ion and as a result, will increase the strength of the acid. Thus, groups having +I effect will decrease the strength of the acids and groups having -I effect will increase the strength of the acids. In the given compounds, -CH3 group has +I effect and Br- group has -I effect. Thus, acids containing Br- are stronger.
The -I effect grows weaker as distance increases. Hence, CH3CH(Br)CH2COOH is a weaker acid than CH3CH2CH(Br)COOH.
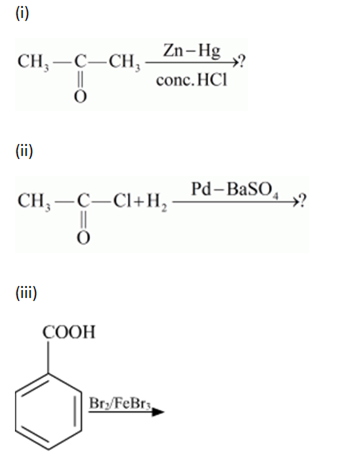
Write the structures of A, B, C, D and E in the following reactions:
Or
(a)Write the chemical equation for the reaction involved in Cannizzaro reaction.
(b)Draw the structure of the semicarbazone of ethanal.
(c)Why pKa of F-CH2-COOH is lower than that of Cl−CH2−COOH?
(d)Write the product in the following reaction:
(e)How can you distinguish between propanal and propanone?
