 Long Answer Type
Long Answer Typei) Mn Shows the highest oxidation state of +7 with oxygen but with fluorine it shows the highest oxidation state of +4.
ii) Cr2+ is a strong reducing agent.
iii) Cu2+ salts are coloured while Zn2+ salts are white.
b) Complete the following equations: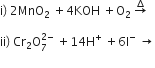
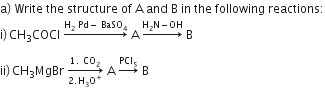
Distinguish between:
i) C6H5-COCH3 and C6H5-CHO
ii) CH3COOH and HCOOH
c) Arrange the following in the increasing order of their boiling points:
CH3CHO,CH3COOH,CH3CH2OH
Or
a) Write the chemical reaction involved in Wolff Kishner reduction.
b) Arrange the following in the increasing order of their reactivity towards nucleophilic addition reaction:
C6H5COCH3, CH3-CHO, CH3COCH3
c) why carboxylic acid does not give reactions of carbonyl group ?
d) Write the product in the following reaction![]()
e) A and B are two functional isomers of compound C3H6O. On heating with NaOH and I2, isomers B forms a yellow precipitate of iodoform whereas isomer A does not form any precipitate. Write the formulae of A and B.
 Short Answer Type
Short Answer TypeRearrange the compounds of each of the following sets in order of reactivity towards SN2 displacement:
(i) 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 3-Bromo-2-methylbutane
(iii) 1-Bromobutane, 1-Bromo-2, 2-dimethylpropane, 1-Bromo-2-methylbutaneHow would you obtain the following?
(i) Benzoquinone from phenol
(ii) 2-methyl propan-2-ol from methyl-magnesium bromide
(iii) Propane-2-ol from propene
(i) Phenol is oxidised to benzoquinone as
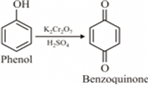
It is an oxidation reaction. The ready oxidation of phenol is explained by the fact that the presence of OH group on the benzene ring increases electron density to the ring due to resonance which makes it for the oxidation reaction.
(ii)

(iii) If the propene is allowed to react with water in the presence ofan acid as catalyst, then propan-2-ol is obtained.
![]()
 Long Answer Type
Long Answer Type(a) Illustrate the following name reactions:
(i) Cannizzaro’s reaction
(ii) Clemmensen reduction
(b) How would you obtain the following?
(i) But-2-enal from ethanal
(ii) Butanoic acid from butanol
(iii) Benzoic acid from ethylbenzene
OR
(a) Given chemical tests to distinguish between the following:
(i) Benzoic acid and ethyl benzoate
(ii) Benzaldehyde and acetophenone
(b) Complete each synthesis by giving missing reagents or products in the following: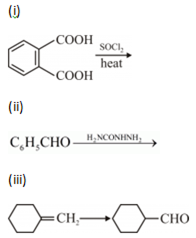
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type(a) Write the products formed when CH3CHO reacts with the following reagents:
(i) HCN
(ii) H2N−OH
(iii) CH3CHO in the presence of dilute NaOH
(b) Give simple chemical tests to distinguish between the following pairs of compounds:
(i) Benzoic acid and Phenol
(ii) Propanal and Propanone
OR
(a) Account for the following:
(i) Cl−CH2COOH is a stronger acid than CH3COOH.
(ii) Carboxylic acids do not give reactions of the carbonyl group.
(b) Write the chemical equations to illustrate the following name reactions:
(i) Rosenmund reduction
(ii) Cannizzaro's reaction
(c) Out of CH3CH2−CO−CH2−CH3 and CH3CH2−CH2−CO−CH3, which gives iodoform test?
 Short Answer Type
Short Answer TypeDo the following conversions in not more than two steps :
Benzoic acid to benzaldehyde
