 Short Answer Type
Short Answer TypeArrange the following in the decreasing order of their basic strength in aqueous solutions:
CH3NH2, (CH3)2NH, (CH3)3 N and NH3
(a) Complete the following chemical reactions equations:
(i) P4+SO2Cl2 -->
(ii) XeF6+H2O -->
(b) Predict the shape and the asked angle (90° or more or less) in each of the following cases:
(i) and the angle O - S - O
(ii) ClF3 and the angle F - Cl - F
(iii) XeF2 and the angle F - Xe - F
Complete the following chemical equations:
(i) NaOH+Cl2 -->
(ii) XeF4+O2F2--->
(b) Draw the structures of the following molecules:
(i) H3PO2
(ii) H2S2O7
(iii) XeOF4
State reasons for each of the following:
The N-O bond in is shorter than the N-O bond in
The shorter N - O bond in is due to the existence of resonance in. The resonating structures can be drawn as follows.

Due to resonance in, the two bonds are equivalent. This leads to a decrease in bond length.
Thus, the N - O bond length in resembles a double bond.
Now, the resonating structures for can be drawn as:
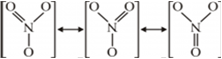
As seen from the above resonating structures of ![]() , the three oxygen atoms are sharing two single bonds and one double bond. So, the real N - O bond length resembles a single bond closely.
, the three oxygen atoms are sharing two single bonds and one double bond. So, the real N - O bond length resembles a single bond closely.
In ![]() the lone pair is delocalized between the 2 oxygen groups. Bond order equal to 1+1/2=3/2.
the lone pair is delocalized between the 2 oxygen groups. Bond order equal to 1+1/2=3/2.
In ![]() lone pair shared between three oxygen atom hence bond order =1+1/3=4/3.
lone pair shared between three oxygen atom hence bond order =1+1/3=4/3.
Greater the bond order shorter the bond. Hence bond length of ![]() less than that of
less than that of ![]()
This explains the existence of shorter bond length of the N - O bond ![]() in than in
in than in ![]() .
.
State reasons for each of the following:
(i) All the P-Cl bonds in PCl5 molecule are not equivalent.
(ii) Sulphur has a greater tendency for catenation than oxygen.
 Long Answer Type
Long Answer Type(i) NF3 is an exothermic compound whereas NCl3 is not.
(ii) F2 is most reactive of all the four common halogens.
(b) Complete the following chemical equations:
(i) C + H2SO4 (conc.)-->
(ii) P4 + NaOH + H2O-->
(iii) Cl2+F2 ------>
(excess)
(a) Account for the following:
(i) The acidic strength decreases in the order HCl > H2S > PH3
(ii) Tendency to form pentahalides decreases down the group in group 15 of the periodic table.
(b) Complete the following chemical equations:
(i) P4 + SO2Cl2-->
(ii) XeF2 + H2O--->
(iii) I2+HNO3(conc.)--->
