 Short Answer Type
Short Answer TypeArrange the following in increasing order of their basic strength:
(i) C6H5 – NH2, C6H5 – CH2 – NH2, C6H5 – NH – CH3
(i) C6H5-NH2 < C6H5-NH-CH3 < C6H5-CH2-NH2
Reason:
C6H5-NH2 will be least basic because of the delocalization of the lone pair of electrons present on the N-atom over the benzene ring due to the ‒R effect of the C6H5 group. However, C6H5-CH2-NH2 will be more basic than C6H5-NH-CH3 because of the electron-releasing nature of the CH3- a group that increases the electron density on the N-atom, making the lone pair of electrons on the N-atom easily available for donation to a proton. The basicity of C6H5-NH-CH3 will be intermediate of C6H5-NH2 and C6H5-CH2-NH2 because the C6H5- the group will tend to pull the electron density from the N-atom. On the other hand, the CH3- group will tend to increase the electron density on the N-atom. Thus, the basic strength of the given amines will follow the above-mentioned order.
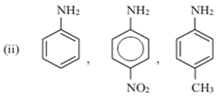
(ii) 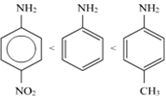
Reason:
The -CH3 group will increase the electron density on the benzene ring because of it is +I effect, while the NO2 group will decrease the electron density on the benzene ring because of its ‒I effect. Hence, the C6H5-NH2 molecule having - the CH3 group will be more basic than C6H5-NH2. Also, it will be more basic than the C6H5-NH2 molecule with the NO2 group.
Give reasons for the following:
(i) N2 is less reactive at room temperature.
(ii) H2Te is the strongest reducing agent amongst all the hydrides of Group 16 elements.
(iii) Helium is used in diving apparatus as a diluent for oxygen.
Give reasons for the following:
(i) Oxygen is a gas but sulphur is solid.
(ii) O3 acts as a powerful oxidising agent.
(iii) BiH3 is the strongest reducing agent amongst all the hydrides of Group 15 elements.
Explain the following facts giving appropriate reason in each case:
(i) NF3 is an exothermic compound whereas NCl3 is not.
(ii) All the bonds in SF4 are not equivalent.
 Long Answer Type
Long Answer Type(a) Draw the molecular structure of the following compounds.
(i) N2O5
(ii) XeOF4
(b) Explain the following observation:
(i) Sulphur has a greater tendency for catenation than oxygen.
(ii) ICI is more reactive than I2.
(iii) Despite the lower value of its electron gain enthalpy with a negative sign, fluorine (F2) is a stronger oxidizing agent than Cl2.
(a) Complete the following chemical equation
(i) Cu + HNO3 (dilute) --->
(ii) XeF4 + O2F2 -->
(b) Explain the following observation:
(i) Phosphorus has a greater tendency for catenation than nitrogen.
(ii) Oxygen is a gas but sulphur a solid.
(iii) The halogens are coloured. Why?
(a) Account for the following:
(i)Ozone is thermodynamically unstable.
(ii)Solid PCl5 is ionic in nature.
(iii)Fluorine forms only one oxoacid HOF.
(b) Draw the structure of
(i) BrF5
(ii) XeF4
OR
(i)Compare the oxidizing action of F2 and Cl2 by considering parameters such as bond dissociation enthalpy, electron gain enthalpy and hydration enthalpy.
(ii)Write the conditions to maximize the yield of H2SO4 by contact process.
(iii)Arrange the following in the increasing order of property mentioned:
(a)H3PO3, H3PO4, H3PO2 (Reducing character)
(b)NH3, PH3, AsH3, SbH3, BiH3 (Base strength)
