 Short Answer Type
Short Answer TypeThe chemistry of corrosion of iron is essentially an electrochemical phenomenon. Explain the reactions occurring during the corrosion of iron in the atmosphere.
The process of corrosion is a redox reaction and involves simultaneous oxidation and reduction reactions. It can, therefore, be referred to as an electrochemical reaction.
In the process of corrosion, due to the presence of air and moisture, oxidation takes place at a particular spot of an object made of iron. That spot behaves as the anode. The reaction at the anode is can be written as follows.
Anode reaction: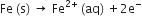
Electrons released at the anodic spot move through the metallic object and go to another spot of the object. There, in the presence of H+ ions, the electrons reduce molecular oxygen. This spot behaves as the cathode. These H+ ions come either from H2CO3, which are formed due to the dissolution of carbon dioxide from the air into water or from the dissolution of other acidic oxides from the atmosphere in water.
The reaction corresponding at the cathode is written as follows.
Cathode reaction:
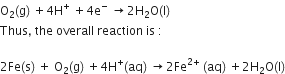
Also, ferrous ions are further oxidised by atmospheric oxygen to ferric ions.
These ferric ions combine with moisture, present in the surroundings, to form a hydrated ferric oxide (Fe2O3, x H2O) i.e., rust.
State reasons for each of the following:
The N-O bond in is shorter than the N-O bond in
State reasons for each of the following:
(i) All the P-Cl bonds in PCl5 molecule are not equivalent.
(ii) Sulphur has a greater tendency for catenation than oxygen.
