 Short Answer Type
Short Answer TypeExplain the following giving an appropriate reason in each case.
O2 and F2 both stabilise higher oxidation states of metals but O2 exceeds F2 in doing so.
Explain the following giving an appropriate reason in each case.
Structures of Xenon fluorides cannot be explained by Valence Bond approach.
Tungsten crystallizes in the body-centred cubic unit cell. If the edge of the unit cell is 316.5 pm, what is the radius of tungsten atom?
Iron has a body centred cubic unit cell with a cell dimension of 286.65 pm. The density of iron is 7.874 g cm-3. Use this information to calculate Avogadro's number (At. Mass of Fe = 55.845 u)
Calculate the amount of KCl which must be added to 1 kg of water so that the freezing point is depressed by 2K. (Kf for water = 1.86 K kg mol-1)
For the reaction
2NO(g) + Cl2(g) --> 2NOCl(g)
The following data were collected. All the measurements were taken at 263 K:
|
Experiment No. |
Initial [NO] (M) |
Initial [Cl2] (M) |
Initial rate of disappearance of Cl2(M/min) |
|
1 |
0.15 |
0.15 |
0.60 |
|
2 |
0.15 |
0.30 |
1.20 |
|
3 |
0.30 |
0.15 |
2.40 |
|
4 |
0.25 |
0.25 |
? |
(a) Write the expression for rate law.
(b) Calculate the value of rate constant and specify its units.
(c) What is the initial rate of disappearance of Cl2 in exp. 4?
How would you account for the following?
i) Many of the transition elements are known to form interstitial compounds.
ii)The metallic radii of the third (5d) series of transition metals are virtually the same as those of the corresponding group member of the second (4d) series.
iii) Lanthanoids from primarily +3 ions, while the actinoids usually have higher oxidation states in their compounds, +4 or even + 6 being typical.
(i) Formation of the interstitial compounds: Transition elements exist in CCP and HCP structure which are known to possess vacant position (Holes),such that transition elements form a few interstitial compounds with elements possessing small atomic radii, like hydrogen, carbon boron, and nitrogen. Small atoms of these type of elements get entrapped in between the void spaces (called as interstices) of the metal lattice. Some of the characteristics of the interstitial compound. These compounds show basically the same chemical properties as the parent metals but vary in the physical properties such as density and hardness. For example, the best known is tungsten carbide WC that is extremely hard and used in steel cutting tools, armour and jewellery.
Explanation: Interstitial compounds are hard and dense in nature. This is because; the smaller atoms of the lighter elements occupy the interstices in the lattice, leading to the much closely packed structure. Because of greater electronic interactions, the strength of metallic bonds also increases.
(ii) Metallic radii of third (5d) series of transition metals are virtually same as those of (4d) series because of the lanthanoid contraction.
Lanthanoid contraction: it is the phenomenon occurring in the 3rd series of the transition element. As they have electron filling up the 4f shell which is filled with the 5d series. The 4f shell has very weak shielding effect and is highly attracted towards the Nucleus due to high nuclear charge so the filling of 4f orbitals before 5d orbital resulting in their atomic radius which is very similar to the elements of the 2nd series i.e. Zr=160 atomic radius and Hf=159 atomic radius.
(iii) The wide range of oxidation states of actinoids is attributed to the fact that the 5f, 6d and 7senergy levels are comparable energies. Therefore all these three sub shells can participate. But the most common oxidation state of actinoids is +3.
Give the formula of each of the following coordination entities:
(i) Co3+ Ion is bound to one Cl-1 , one NH3 molecule and two bidentate ethylenediamine (en) molecules.
(ii) Ni2+ ion is bound to two water molecules and two oxalate ions. Write the name and magnetic behavior of each of the above coordination entities.
(At. No. Co = 27, Ni = 28)
a) What type of a battery is the lead storage battery? Write the anode and the cathode reactions and the overall reaction occurring in a lead storage battery when current is drawn from it.
(b) In the button cell, widely used in watches, the following reaction takes place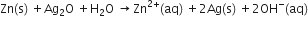
Determine E° and G° for the reaction 
