 Short Answer Type
Short Answer TypeExplain the following giving an appropriate reason in each case.
O2 and F2 both stabilise higher oxidation states of metals but O2 exceeds F2 in doing so.
Explain the following giving an appropriate reason in each case.
Structures of Xenon fluorides cannot be explained by Valence Bond approach.
Tungsten crystallizes in the body-centred cubic unit cell. If the edge of the unit cell is 316.5 pm, what is the radius of tungsten atom?
Iron has a body centred cubic unit cell with a cell dimension of 286.65 pm. The density of iron is 7.874 g cm-3. Use this information to calculate Avogadro's number (At. Mass of Fe = 55.845 u)
Calculate the amount of KCl which must be added to 1 kg of water so that the freezing point is depressed by 2K. (Kf for water = 1.86 K kg mol-1)
For the reaction
2NO(g) + Cl2(g) --> 2NOCl(g)
The following data were collected. All the measurements were taken at 263 K:
|
Experiment No. |
Initial [NO] (M) |
Initial [Cl2] (M) |
Initial rate of disappearance of Cl2(M/min) |
|
1 |
0.15 |
0.15 |
0.60 |
|
2 |
0.15 |
0.30 |
1.20 |
|
3 |
0.30 |
0.15 |
2.40 |
|
4 |
0.25 |
0.25 |
? |
(a) Write the expression for rate law.
(b) Calculate the value of rate constant and specify its units.
(c) What is the initial rate of disappearance of Cl2 in exp. 4?
How would you account for the following?
i) Many of the transition elements are known to form interstitial compounds.
ii)The metallic radii of the third (5d) series of transition metals are virtually the same as those of the corresponding group member of the second (4d) series.
iii) Lanthanoids from primarily +3 ions, while the actinoids usually have higher oxidation states in their compounds, +4 or even + 6 being typical.
Give the formula of each of the following coordination entities:
(i) Co3+ Ion is bound to one Cl-1 , one NH3 molecule and two bidentate ethylenediamine (en) molecules.
(ii) Ni2+ ion is bound to two water molecules and two oxalate ions. Write the name and magnetic behavior of each of the above coordination entities.
(At. No. Co = 27, Ni = 28)
a) What type of a battery is the lead storage battery? Write the anode and the cathode reactions and the overall reaction occurring in a lead storage battery when current is drawn from it.
(b) In the button cell, widely used in watches, the following reaction takes place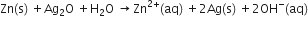
Determine E° and G° for the reaction 
A lead storage battery has a secondary cell. Thus, it can be recharged by passing direct current through it. Therefore, it can be reused. It is used in automobiles.
In a lead storage cell, the anode is made of spongy lead and the cathode is a grid of lead packed with lead dioxide. The electrolyte used is H2SO4.

