 Long Answer Type
Long Answer Type(a) Define electric dipole moment. Is it a scalar or a vector? Derive the expression for the electric field of a dipole at a point on the equatorial plane of the dipole.
(b) Draw the equipotential surfaces due to an electric dipole. Locate the points where the potential due to the dipole is zero.
Using Gauss’ laws deduce the expression for the electric field due to a uniformly charged spherical conducting shell of radius R at a point (i) outside and (ii) inside the shell.
Plot a graph showing variation of electric field as a function of r > R and r < R. (r being the distance from the centre of the shell).
Using Bohr’s postulates, derive the expression for the frequency of radiation emitted when electron in hydrogen atom undergoes transition from higher energy state (quantum number ni ) to the lower state, (nf ).
When electron in hydrogen atom jumps from energy state ni =4 to nf =3, 2, 1, identify the spectral series to which the emission lines belong.
According to Bohr’s postulates, in a hydrogen atom, a single electron revolves around a nucleus of charge +e. For an electron moving with a uniform speed in a circular orbit on a given radius, the centripetal force is provided by the Coulomb force of attraction between the electron and the nucleus.
Therefore, 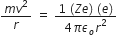 ... (1)
... (1) 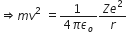
So, Kinetic Energy, K.E = 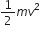
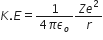
Potential energy is given by, P.E = 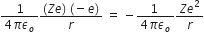
Therefore, total energy is given by, E = K.E + P.E = 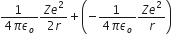
E = 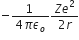 , is the total energy.
, is the total energy.
For nth orbit, E can be written as En,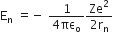 ... (2)
... (2)
Now, using Bohr's postulate for quantization of angular momentum, we have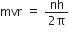
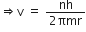
Putting this value of v in equation (1), we get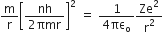
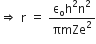
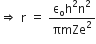
Now, putting value of rn in equation (2), we get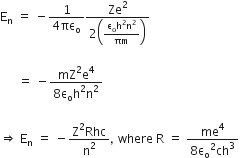
R is the rydberg constant.
For hydrogen atom Z =1,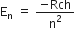
If ni and nf are the quantum numbers of initial and final states and Ei & Ef are energies of electron in H-atom in initial and final state, we have 

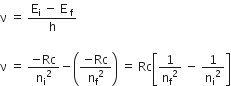
That is, when electron jumps from ni = 4 to nf = 3.21 .
Radiation belongs to Paschen, Balmer, and Lyman series.
(a) Draw the plot of binding energy per nucleon (BE/A) as a function of mass number A. Write two important conclusions that can be drawn regarding the nature of nuclear force.
(b) Use this graph to explain the release of energy in both the processes of nuclear fusion and fission.
(c) Write the basic nuclear process of neutron undergoing –decay. Why is the detection of neutrinos found very difficult?
