 Multiple Choice Questions
Multiple Choice QuestionsThe correct set of four quantum numbers for the valence electrons fo rubidium atom (Z = 37) is
5,0,0, +1/2
5,1,0,6+1/2
5,1,1,+1/2
5,1,1,+1/2
If Z is a compressibility factor, Vander Waal's equation at low pressure can be written as
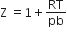
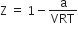
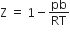
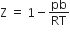
For the complete combustion of ethanol, C2H5OH (l) + 3O2 (g) → 2CO2 (g) + 3H2O (l), the amount of heat produced as measured in a bomb calorimeter, is 1364.47 kJ mol-1 at 25oC. Assuming ideality the enthalpy of combustion, ∆CH, for the reaction will be (R = 8.314 JK-1 mol-1)
-1366.95 kJ mol-1
-1361.95 kJ mol-1
-1460.50 kJ mol-1
-1460.50 kJ mol-1
For the reaction,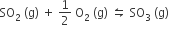
if Kp = Kc (RT)x where the symbol has usual meaning then the value of x is (assuming ideality)
-1
-1/2
1/2
1/2
In which of the following reactions H2O2 acts as a reducing agent?
I. H2O2 + 2H+ + 2e- →2H2O
II. H2O2 - 2e- →O2 + 2H+
III. H2O2 +2e-→ 2OH-
IV. H2O2+ 2OH- -2e- →O2 + 2H2O
I and II
III and IV
I and III
I and III
The correct statement for the molecule, CsI3 is
It is a covalent molecules
It contains Cs+ and I3-
It contains Cs3+ and I- ions
It contains Cs3+ and I- ions
For the estimation of nitrogen 1.4g of an organic compound was digested by Kjeldahl's method and the evolved ammonia was absorbed in 60 mL of M/10 sulphuric acid. The unreacted acid required 20 mL of M/10 sodium hydroxide for complete neutralisation. The percentage of nitrogen in the compound is
6%
10%
3%
3%
The resistance of 0.2 M solution of an electrolyte is 50 Ω. The specific conductance of the solution of 0.5 M solution of the same electrolyte is 1.4 S m-1 and resistance of the same solution of the same electrolyte is 280 Ω. The molar conductivity of 0.5 M solution of the electrolyte in Sm-2 mol-1 is
5 x 10-4
5 x 10-3
5 x 103
5 x 103
The equivalent conductance of NaCl at concentration C at infinite dilution are λC and λ∞ respectively. The correct relationship between λC and λ∞ is given as (where the constant B is positive)
λC = λ∞ +(B)C
λC = λ∞ -(B)C
λC = λ∞ -(B)
λC = λ∞ -(B)