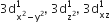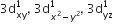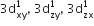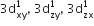Multiple Choice Questions
Multiple Choice QuestionsCopper sulphates dissolves in excess of KCN to give:
CuCN
[Cu(CN)4]3-
[Cu(CN)4]2-
[Cu(CN)4]2-
In which of the following pairs are both the ions coloured in aqueous solution?
(At. no.: Sc = 21, Ti = 22, Ni = 28, Cu = 29, Co = 2)
Ni2+, Ti3+
Sc3+, Ti3+
Sc3+,Co2+
Sc3+,Co2+
Al2O3 can be converted to anhydrous AlCl3 by heating:
Al2O3 with HCl gas
Al2O3 with NaCl in solid state
a mixture of Al2O3 and carbon in dry Cl2 gas
a mixture of Al2O3 and carbon in dry Cl2 gas
The appearance of colour is solid alkali metal halides is generally due to:
F- centres
Schottky defect
Frenkel defect
Frenkel defect
If   the standard emf of the reaction:
 the standard emf of the reaction:
Fe + 2 Fe3+ →3Fe2+
will be:
0.330 V
1.653 V
C.
Given that,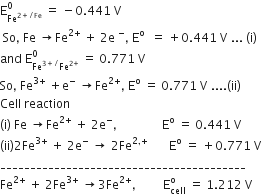
So, on the basis of cell reaction following half-cell reactions are written
At anode:
Fe → Fe2+ + 2e-    (oxidation)
At cathode:
2Fe3+ + 2e- →2Fe2+ (reduction)
So,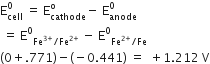
For the reactionÂ
2A + B → 3C + D
Which of the following does not express the reaction rate?

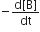


Consider the reaction
N2 (g) + 3 H2 (g) → 2 NH3 (g)Â
The equality relationship between   is:
 is:




Which of the following is not chiralÂ
2-butanol
2,3 -dibromo pentane
3- bromopentane
3- bromopentane
[Co(NH3)4(NO2)2]Cl exhibits:
linkage isomerism, ionisation isomerism and optical isomerism
Linkage isomerism, ionisation isomerism and geometrical isomerism
ionization isomerism, geometrical isomerism and optical isomerism
ionization isomerism, geometrical isomerism and optical isomerism
[Cr(H2O)6]Cl3 (at. no. of Cr = 24) has a magnetic moment of 3.83 BM, the correct distribution of 3d electrons in the chromium of the complex is: