 Multiple Choice Questions
Multiple Choice QuestionsDuring the process of digestion, the proteins present in food materials are hydrolysed in amino acids. The two enzymes involved in the process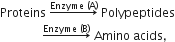
are respectively:
amylase and maltase
diastase and lipase
pepsin and trypsin
pepsin and trypsin
[NH(CH2)NHCO(CH2)4CO]n
co-polymer
addition polymer
thermosetting polymer
thermosetting polymer
A carbonyl compound reacts with hydrogen cyanide to form cyanohydrin which on hydrolysis forms a racemic mixture of alpha-hydroxy acid. The carbonyl compound is:
acetaldehyde
acetone
diethyl ketone
diethyl ketone
Which one of the following is a peptide hormone?
Glucagon
Testosterone
Thyroxin
Thyroxin
The major organic product in the reaction,
CH3 -O- CH(CH3)2 + HI → Product is:
CH3OH + (CH3)2 + CHI
ICH2OCH(CH3)2
CH3O CI(CH3)2
CH3O CI(CH3)2
A carbonyl compound reacts with hydrogen cyanide to form cyanohydrin which on hydrolysis forms a racemic mixture of alpha -hydroxy acid. The carbonyl compound is:
CH3-CH2-CH2COCH3
(CH3)2C=O
CH3CH2CHO
CH3CH2CHO
D.
CH3CH2CHO
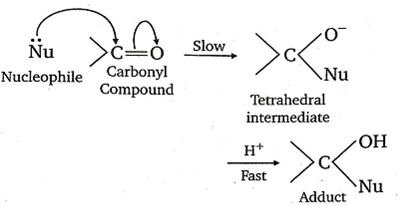
The carbonyl compounds undergo nucleophilic addition reaction because oxygen is more electronegative than carbon. As such, it withdraws share pi electron pair towards itself and gets a partial negative charge, therefore carbon gets a partial positive charge and becomes susceptible to nucleophilic attack.
The aldehyde is more reactive than ketones towards nucleophiles. This can be explained on the basis of inductive effect as well as steric effect. The addition of nucleophiles is based upon the positive charge present on a carbon atom of > C =O group.
In aldehyde >C=O group is present at least one alkyl group (except formaldehyde) which has +I effect (electron donating effect) and which decreases the positive charge of carbon, thereby making the attack to nucleophile difficult. The nucleophilic attack becomes more difficult in ketones having a minimum of two alkyl groups.
Hence, by means of attachment of alkyl groups (due to +I effect) rate of nucleophiles addition decreases.
Order of +I effect in an alkyl group.![]()
order of nucleophilic addition in given carbonyl compound is
CH3CHO > CH3-CH2- CHO > (CH3)2CO> CH3CH2CH2COCH3
Self-condensation of two moles of ethyl acetate in presence of sodium ethoxide yields
ethyl butyrate
acetoacetic ester
methyl acetoacetate
methyl acetoacetate
Ethylene oxide when treated with Grignard reagent yields:
Secondary alcohol
tertiary alcohol
cyclopropyl alcohol
cyclopropyl alcohol
Which of the following is more basic than aniline?
Diphenylamine
Triphenylamine
p-nitroaniline
p-nitroaniline
