 Multiple Choice Questions
Multiple Choice QuestionsGive that the equilibrium constant for the reaction,
2SO2 (g) + O2 (g) ⇌ 2SO3 (g)
has a value of 278 at a particular temperature. What is the value of the equilibrium constant for the following reaction t the same temperature?
SO3 (g) ⇌ SO2 (g) +1/2 O2 (g)
1.8 x 10-3
3.6 x 10-3
6.0 x 10-2
6.0 x 10-2
Given the reaction between two gases represented by A2 and B2 to give the compound AB (g).
A2(g) +B2 (g) ⇌ 2AB (g)
At equilibrium the concentration
of A2 = 3.0 x 10-3 M
of B2 = 4.2 x 10-3 M
of AB = 2.8 x 10-3 M
If the reaction takes place in a sealed vessel at 527oC, then the value of Kc will be
2.0
1.9
0.62
0.62
During the change of O2 to O2- ion, the electron adds on which one of the following orbitals?
π* orbital
π orbital
σ* orbital
σ* orbital
A certain gas takes three times as long to effuse out as helium. Its molecular mass will be
27 u
36 u
64 u
64 u
For real gases van der Waal's equation is written as,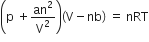
where, a and b are van der Waal's constants.
Two sets of gases are
I. O2, CO2, H2 and He
II. CH4, O2, and H2
The gases given in set- I increasing order of 'b' and gases given in set -II in decreasing order of 'a' are arranged below. Select the correct order from the following.
(I) He < H2 < CO2 < O2
(II) CH4> H2> O2
(I) O2 < He < H2 < CO2
(II) H2> O2 > CH4
(I) O2 < He < O2 < CO2
(II) CH4 > O2 > H2
(I) O2 < He < O2 < CO2
(II) CH4 > O2 > H2
Equal volumes of two monoatomic gases, A and B, at same temperature and pressure are mixed. The ratio of specific heats (Cp/Cv) of the mixture will be
0.83
1.50
3.3
3.3
Four diatomic species are listed below. Identify the correct order in which the bond order is increasing in them.
NO< O2-<C22- < He2+
O2- <NO< C22- < He2+
C22- < He2+ <O2- <NO
C22- < He2+ <O2- <NO
In the replacement reaction![]()
The reaction will be most favourable if M happens to be
Na
K
Rb
Rb
A structure of a mixed oxide is cubic close packed (ccp). The cubic unit cell of mixed oxide is composed of oxide ions. One fourth of the tetrahedral voids are occupied by divalent metal A and the octahedral voids are occupied by a monovalent metal B. The formula of the oxide is
ABO2
A2BO2
A2B3O4
A2B3O4