Long Answer Type
Long Answer Type(a) Define the terms osmosis and osmotic pressure. Is the osmotic pressure of a solution a colligative property? Explain.
(b) Calculate the boiling point of a solution prepared by adding 15.00 g of NaCl to 250.0 g of water. (Kb for water = 0.512 K kg mol-1), (Molar mass of NaCl = 58.44 g)
(i) NF3 is an exothermic compound whereas NCl3 is not.
(ii) F2 is most reactive of all the four common halogens.
(b) Complete the following chemical equations:
(i) C + H2SO4 (conc.)-->
(ii) P4 + NaOH + H2O-->
(iii) Cl2+F2 ------>
(excess)
(a) Account for the following:
(i) The acidic strength decreases in the order HCl > H2S > PH3
(ii) Tendency to form pentahalides decreases down the group in group 15 of the periodic table.
(b) Complete the following chemical equations:
(i) P4 + SO2Cl2-->
(ii) XeF2 + H2O--->
(iii) I2+HNO3(conc.)--->
(i) The acidity of a molecule depends on the polarity of the bond between central atom and the hydrogen atom. Greater the polarity higher will be the acidity.
And the polarity of the bond depends on the electronegativity of the central atom. In a period, the electronegativity decreases in the order Cl > S > P. As a result, the loss of H+ ions decreases.
Thus, the acidic strength of the hydrides decreases in the
Following order:
HCl > H2S > PH3
(ii) Nitrogen does not form pentahalide because it does not have d-orbital. P, As, Sb form pentahalide. Bi does not form pentahalide. The tendency to form pentahalide decrease down the group. This because of inert pair effect.
Due to the inert pair effect, ns2 electron remains inert in a chemical reaction and element shows -2 oxidation state. Inert pair effect increases down the group. Thus the tendency to form pentahalides decrease down the group 15.
(b)
(i) P4 + 10SO2Cl2---> 4PCl5 + 10SO2
(ii) 2XeF2 + 2H2O----> 2Xe + 4HF + O2
(iii) I2 + 10HNO3--> 2HIO3+10NO2+4H2O
Answer the following:
(i) Haloalkanes easily dissolve in organic solvents, why?
(ii) What is known as a racemic mixture? Give an example.
(iii) Of the two Bromo derivatives, C6H5CH(CH3)Br and C6H5CH(C6H5)Br, which one is more reactive in Sn1 substitution reaction and why?
Describe the following giving one example for each:
(i) Detergents
(ii) Food preservatives
(iii) Antacids
Give chemical tests to distinguish between
(i) Propanal and propanone,
(ii) Benzaldehyde and acetophenone.
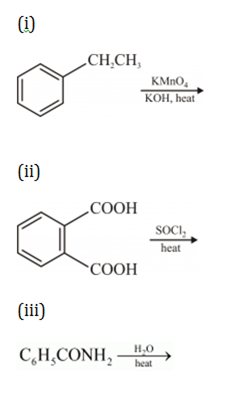
(b) How would you obtain
(i) But-2-enal from ethanal,
(ii) Butanoic acid from butanol,
(iii) Benzoic acid from ethylbenzene?
(a) Describe the following giving linked chemical equations:
(i) Cannizzaro reaction
(ii) Decarboxylation
(b) Complete the following chemical equations: