 Short Answer Type
Short Answer TypeCalculate the emf of the following cell at 25°C:

Given E°cell = + 0.46 V and log 10n = n
The rate of a reaction becomes four times when the temperature changes from 293 K to 313 K. Calculate the energy of activation (Ea) of the reaction assuming that it does not change with temperature.
[R = 8·314 J K-1 mol-1, log 4 = 0·6021]
For the complex [NiCl4]2-, write
(i) The IUPAC name.
(ii) The hybridization type.
(iii) The shape of the complex.
(Atomic no. of Ni = 28)
What is meant by crystal field splitting energy? On the basis of crystal field theory, write the electronic configuration of d4 in terms of t2g and eg in an octahedral field when
(i)![]() 0 > P
0 > P
(ii) ![]() 0 < P
0 < P
Crystal Field Splitting Energy: Crystal field theory was given to explain the structure and stability of the coordination complexes. This theory has some assumption like the metal ion is considered to be a point positive charge and the ligands are negative charge. The complexes are formed mainly by the d- block elements due to their variable oxidation states and variable coordination number. The d- subshell has 5 degenerate orbitals. when the ligand approach to the metal ion, the energy of the degenerate orbitals is increased and further splitting of degenerate orbital takes place into t2g and eg orbital. The difference between the t2g and eg orbitals is called as the crystal field splitting energy.
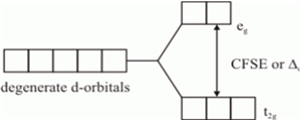
This splitting energy depends on the typo of ligand.
Strong field ligand will have high splitting energy and weak field ligand have low splitting energy.
For electric configuration of d4
(i) When 0 > P
In strong field ligand, the fourth electron will come back and pair in the t2g orbitals. So, the configuration will be Electronic configuration is

(ii) When o < P
In weak field ligand, the Electronic configuration is

Give reasons for the following:
(i) Oxygen is a gas but sulphur is solid.
(ii) O3 acts as a powerful oxidising agent.
(iii) BiH3 is the strongest reducing agent amongst all the hydrides of Group 15 elements.
