 Short Answer Type
Short Answer TypeCalculate the emf of the following cell at 25°C:

Given E°cell = + 0.46 V and log 10n = n
The rate of a reaction becomes four times when the temperature changes from 293 K to 313 K. Calculate the energy of activation (Ea) of the reaction assuming that it does not change with temperature.
[R = 8·314 J K-1 mol-1, log 4 = 0·6021]
For the complex [NiCl4]2-, write
(i) The IUPAC name.
(ii) The hybridization type.
(iii) The shape of the complex.
(Atomic no. of Ni = 28)
What is meant by crystal field splitting energy? On the basis of crystal field theory, write the electronic configuration of d4 in terms of t2g and eg in an octahedral field when
(i)![]() 0 > P
0 > P
(ii) ![]() 0 < P
0 < P
Give reasons for the following:
(i) Oxygen is a gas but sulphur is solid.
(ii) O3 acts as a powerful oxidising agent.
(iii) BiH3 is the strongest reducing agent amongst all the hydrides of Group 15 elements.
(i) Oxygen forms O2 which is a gas and sulphur forms S8 which is solid this can be explained as:
Due to the small size of oxygen, it has less tendency for catenation and the high tendency of pp-pp multiple bonds, hence forms stable O2 molecules whereas sulphur because of its higher tendency for catenation and lesser tendency to form pp-pp multiple bonds forms S8 molecules having 8-membered puckered ring. Held together by strong covalent bonds and exist as a polyatomic molecule, so it exists solid.
(ii) Ozone is not a very stable compound under normal conditions and decomposes readily on heating to give a molecule of oxygen and nascent oxygen. Nascent oxygen, being a free radical, is very reactive.
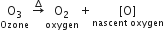
Therefore, O3 acts as powerful oxidising agent.
(iii) BiH3 is the strongest reducing agent amongst all the hydrides of group-15 elements because as we more down the group, the atomic size increases and the stability of the hydrides of group 15 element decreases. Since the stability of hydrides decreases on moving from NH3 to BiH3, the reducing character of the hydrides increases on moving from NH3 to BiH3.
