 Long Answer Type
Long Answer Type(a) Define the following terms:
(i) Ideal solution
(ii) Azeotrope
(iii) Osmotic pressure
(b) A solution of glucose (C6H12O6) in water is labeled as 10% by weight. What would be the molality of the solution?
(Molar mass of glucose = 180 g mol-1)
(a) Give reasons for the following:
(i) Mn3+ is a good oxidising agent.
(ii) ![]() Values are not regular for first row transition metals (3d series).
Values are not regular for first row transition metals (3d series).
(iii) Although ‘F’ is more electronegative than ‘O’, the highest Mn fluoride is MnF4, whereas the highest oxide is Mn2O7.
(b) Complete the following equations:
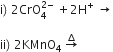
(a) Why do transition elements show variable oxidation states?
(i) Name the element showing the maximum number of oxidation states among the first series of transition metals from Sc (Z = 21) to Zn (Z = 30).
(ii) Name the element which shows only +3 oxidation state.
(b) What is lanthanoid contraction? Name an important alloy which contains some of the lanthanoid metals.
(a) The ability of the transition metal to exhibit variable valency is generally attributed to the availability of more electrons in the (n-1)d orbitals which are closer to the outermost ns orbital in energy levels.
Thus in the case of iron, we get the divalent Fe(II) state when only the 2 electrons in the 4s orbital are removed. And we get the trivalent Fe(III) state when one more 3d electron is removed, in addition to the two 4s electrons from the neutral Fe atom.
(i) Mn shows a maximum number of oxidation states among the first series of transition metals from Sc to Zn. Mn exhibits all the oxidation states from +2 to +7.
(ii) Scandium shows only +3 oxidation state.
(b) The regular decrease in the size of the atoms and ions with increasing atomic number is known as lanthanide contraction. It arises because as we move along the lanthanide series, the nuclear charge increases by one unit at each successive element, the new electron is added into the same subshell (viz., 4f). As a result, the attraction on the electrons by the nucleus increases and this tends to decrease the size. Further, as the new electron is added into the f-subshell, there is imperfect shielding of one electron by another in this subshell due to the shapes of these f-orbitals. This imperfect shielding is unable to counterbalance the effect of the increased nuclear charge. Hence, the net result is a contraction in the size though the decrease is very small.
(a) How will you convert the following?
(i) Propanone to Propan-2-ol
(ii) Ethanal to 2-hydroxy propanoic acid
(iii) Toluene to benzoic acid
(b) Give simple chemical test to distinguish between:
(i) Pentan-2-one and Pentan-3-one
(ii) Ethanal and Propanal
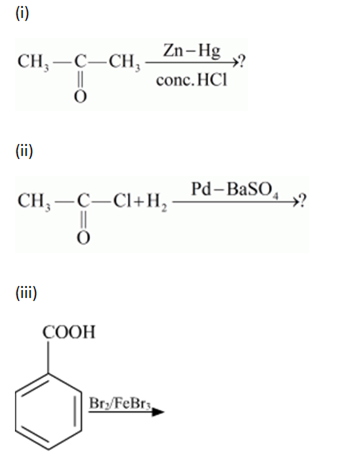
(a) Write the products of the following reactions:
(b) Which acid of each pair shown here would you expect to be stronger?
(i) F — CH2 —COOH or Cl — CH2 — COOH
(ii) 
