 Multiple Choice Questions
Multiple Choice QuestionsThe order of decreasing ease of abstraction of hydrogen atoms in the following molecule
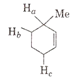
Ha > Hb > Hc
Ha > Hc > Hb
Hb > Ha > Hc
Hc > Hb > Ha
Among the following statements about the molecules X and Y, the one(s) which correct is (are)
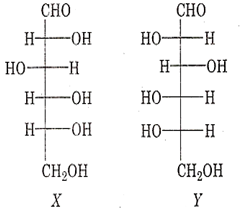
X and Y are diastereomers
X and Y are enantiomers
X and Y are both aldohexoses
X is a D-sugar and Y is an L-sugar
The electronic configuration of Cu is
[Ne] 3s2, 3p6, 3d9, 4s2
[Ne] 3s2, 3p6, 3d10, 4s1
[Ne] 3s2, 3p6, 3d3, 3d9, 4s2, 4p6
[Ne] 3s2, 3p6, 3d5, 4s2, 4p4
B.
[Ne] 3s2, 3p6, 3d10, 4s1
Atomic number of Copper is 29, its electronic configuration is-
Cu29 = 1s2, 2s2, 2p6, 3s2, 3p6, 3d10, 4s1
Since, fully filled orbitals are more stable, so one electron from 4s orbital transfers into 3d orbital. Electronic configuration of Neon is-
Ne10 = 1s2, 2s2, 2p6
Therefore, using inert gas, the configuration of Cu can be represented as-
Cu29 - [Ne] 3s2, 3p6, 3d10, 4s1
The rate of a certain reaction is given by, rate = k [H+]n. The rate increases 100 times when the pH changes from 3 to 1. The order (n) of the reaction is
2
0
1
1.5
The correct statement regarding the following energy diagrams is
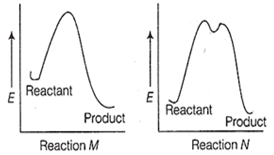
Reaction M is faster and less exothermic than reaction N
Reaction M is slower and less exothermic than reaction N
Reaction M is faster and more exothermic than reaction N
Reaction M is slower and more exothermic than reaction N
