 Multiple Choice Questions
Multiple Choice QuestionsThe order of decreasing ease of abstraction of hydrogen atoms in the following molecule
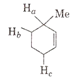
Ha > Hb > Hc
Ha > Hc > Hb
Hb > Ha > Hc
Hc > Hb > Ha
Among the following statements about the molecules X and Y, the one(s) which correct is (are)
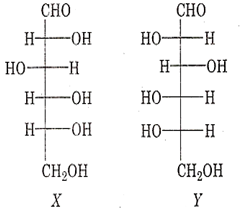
X and Y are diastereomers
X and Y are enantiomers
X and Y are both aldohexoses
X is a D-sugar and Y is an L-sugar
The electronic configuration of Cu is
[Ne] 3s2, 3p6, 3d9, 4s2
[Ne] 3s2, 3p6, 3d10, 4s1
[Ne] 3s2, 3p6, 3d3, 3d9, 4s2, 4p6
[Ne] 3s2, 3p6, 3d5, 4s2, 4p4
The rate of a certain reaction is given by, rate = k [H+]n. The rate increases 100 times when the pH changes from 3 to 1. The order (n) of the reaction is
2
0
1
1.5
The correct statement regarding the following energy diagrams is
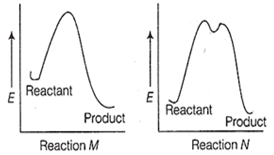
Reaction M is faster and less exothermic than reaction N
Reaction M is slower and less exothermic than reaction N
Reaction M is faster and more exothermic than reaction N
Reaction M is slower and more exothermic than reaction N
C.
Reaction M is faster and more exothermic than reaction N
In reaction M, there is only an intermediate step, so it is a fast reaction. Also, energy of product is much lesser than that of the reactant, as compared to as shown in reaction N. Thus, reaction M is faster and more exothermic than reaction N.
