 Multiple Choice Questions
Multiple Choice QuestionsA metal has a fcc lattice. The edge length of the unit cell is 404 pm. The density of the metal is 2.72 g cm3. The molar mass of the metal is (NA vogadro's constant = 6.02x 1023 mol-1).
40 g mol-1
30 g mol-1
27g mol-1
20 g mol-1
A magnetic moment of 1.73 BM will be shown by one among the following
[Cu(NH3)4]2+
[Ni(CN)4]2-
TiCl4
[CoCl6]4-
Roasting of sulphides gives the gas X as a by-product. This is a colourless gas with choking smell of burnt sulphur. and causes great damage to respiratory organs as a result of acid rain. Its aqueous solution is acidic acts as a reducing agent and its acid has never been insolated. The gas X is
H2S
SO2
CO2
SO3
Which ofthe following lanthanoid ions is diamagnetic?
(At. nos. Ce = 58, Sm = 62, Eu = 63, Yb= 70)
Ce2+
Sm2+
Eu2+
Yb2+
D.
Yb2+
Lanthanoid ion with no unpaired electron is diamagnetic in nature.
Ce58 = [Xe] 4f2 5d0 6s2
Ce2+= [Xe] 4f2 (two unpaired electrons)
Sm62= [Xe] 4f6 5d0 6s2
Sm2+= [Xe] 4f6 (six unpaired electrons)
Eu63 = [Xe] 4f7 5d0 6s2
Eu2+= [Xe] 4f7 (seven unpaired electrons)
Yb70 =[Xe] 4f14 5d0 6s2
Yb2+= [Xe] 4f14 (no unpaired electrons)
Because of the absence of unpaired electrons, Yb2+ is diamagnetic.
Structure of the compound whose IUPAC name is 3-ethyl-2-hydroxy-4-methylhex-3-en-5-ynoic acid is
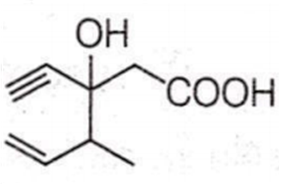
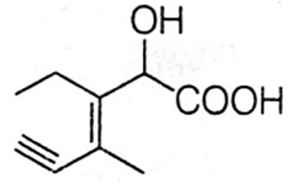
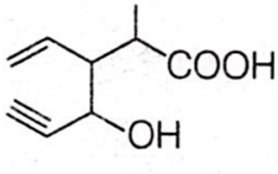
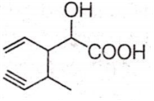
Antiseptics and disinfectants either kill or prevent growth of microorganisms. Identify which of the following is not true.
A 0.2% solution of phenol is an antiseptic while 1% solution acts as a disinfectant.
Chlorine and iodine are used as strong disinfectants.
Dilute solutions of boric acid and hydrogen, peroxide are strong antiseptics.
Disinfectants harm the living tissues.
